Sky Medical Technology's geko NMES Device, USA

Sky Medical Technology's geko NMES Device, USA
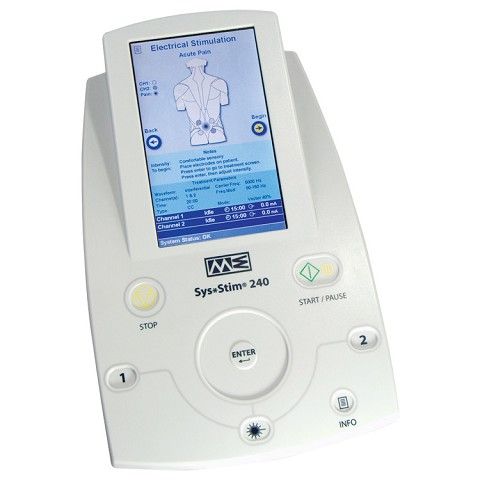
The geko™ device is a neuromuscular electrostimulation (NMES) device designed to enhance blood flow to prevent venous thromboembolism (VTE).

PDF) The geko™ Electro-Stimulation Device for Venous Thromboembolism Prophylaxis: A NICE Medical Technology Guidance

Does Neuromuscular Electrical Stimulation Improve Recovery Following Acute Ankle Sprain? A Pilot Randomised Controlled Trial - Thomas W Wainwright, Louise C Burgess, Robert G Middleton, 2019

Enhancing standard of wound care with innovative medical technology - Med-Tech Innovation
The geko™ T3 Device is an electrical stimulation band that utilises neuromuscular electrical stimulation (NMES) technology. The geko™ T3 Device is

Firstkind Geko device - NMES Neuromuscular Electrostimulation
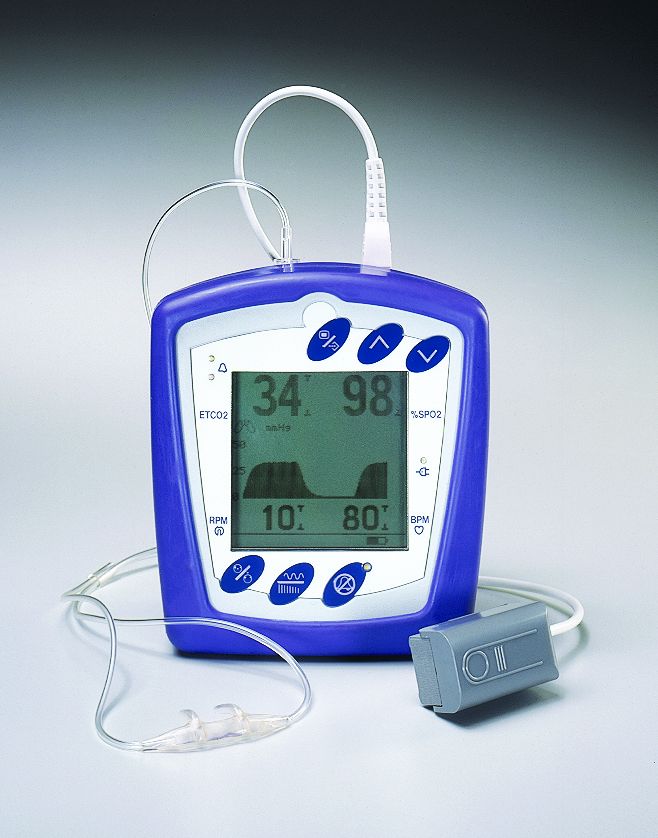
Smiths Medical - BCI Capnocheck II Hand-Held Capnograph - CME Corp

Neuromuscular electrical stimulation via the peroneal nerve is superior to graduated compression socks in reducing perceived muscle soreness following intense intermittent endurance exercise

Sky Medical Technology Announces geko™ Device Is Now Reimbursed in the UK

The geko Device Makes its Debut at Major U.S. Orthopaedic Conference

Sky Medical Technology wins further FDA clearance to market the new (W3) geko™ device variant for venous insufficiency and ischemia

Sky Medical Technology Partner-With-Us – and find out what the geko™ device can do for your patients