FDA Clears Nanowear's SimpleSense Non-Invasive Continuous Blood

FDA Clears Nanowear's SimpleSense Non-Invasive Continuous Blood
Nanowear's remote monitoring device and its SimpleSense platform received FDA 510(k) clearance as a continuous blood pressure monitor.

Inside Precision Medicine (@Inside_PM) / X

Nanowear Announces FDA 510(k) Clearance for AI-enabled Continuous
Digital Technologies to Support Better Outcome and Experience of

FDA 510(k) Clearance — Nanowear
Multiparametric cloth-based wearable, SimpleSense, estimates blood
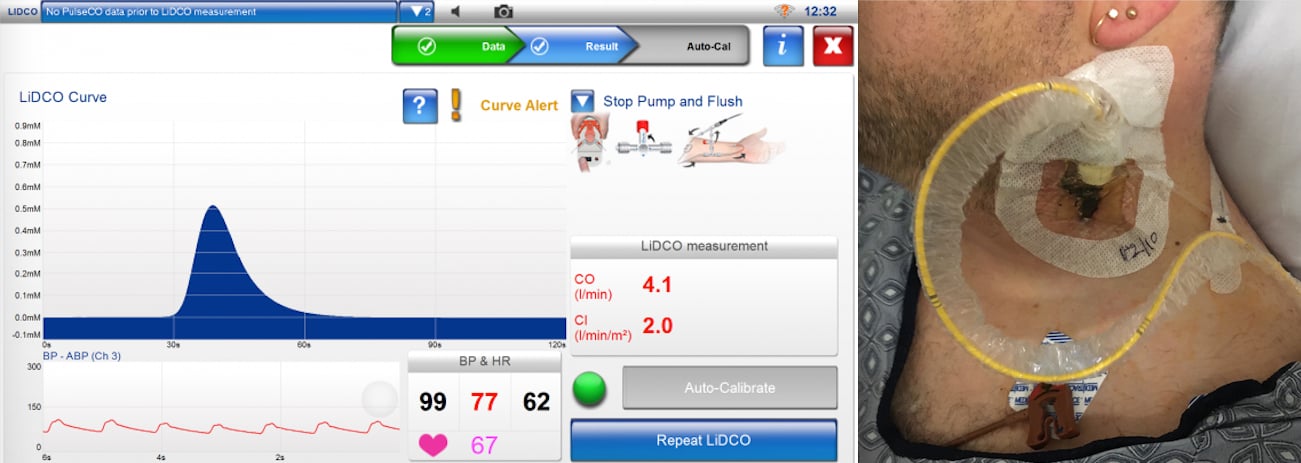
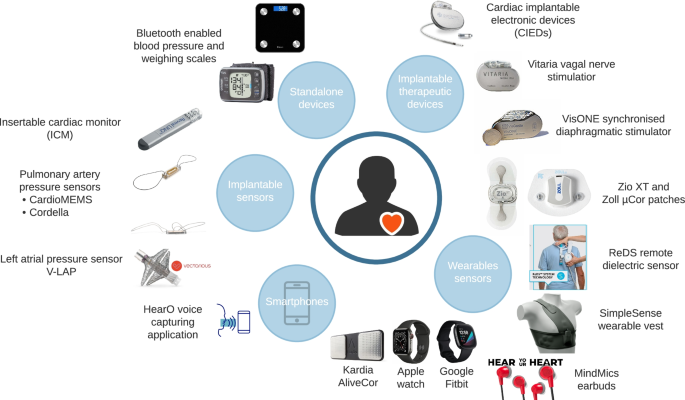
Trends in Cardiac Output Monitoring Device Technologies

FDA 510(k) clearance for SimpleSense-BP

Caretaker Medical Wins CE Certification for Caretaker4 Wireless

FDA 510(k) Clearance — Nanowear
Sensors, Free Full-Text

Nanowear Announces FDA 510(k) Clearance for AI-enabled Continuous

Multiparametric cloth-based wearable, SimpleSense, estimates blood

Nanowear gets FDA nod for wearable blood pressure monitoring system
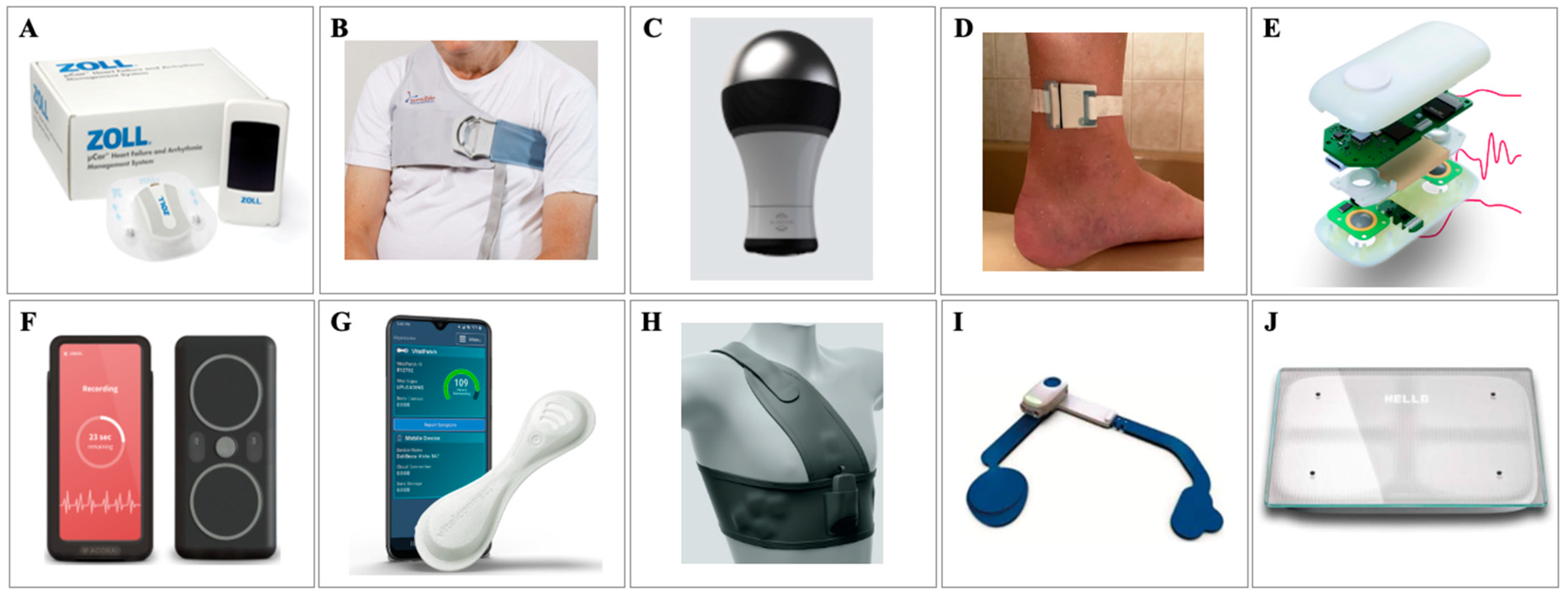
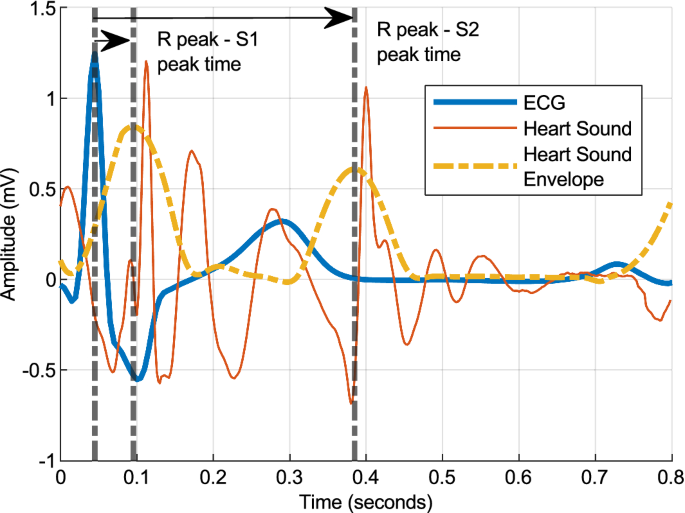
JCM, Free Full-Text
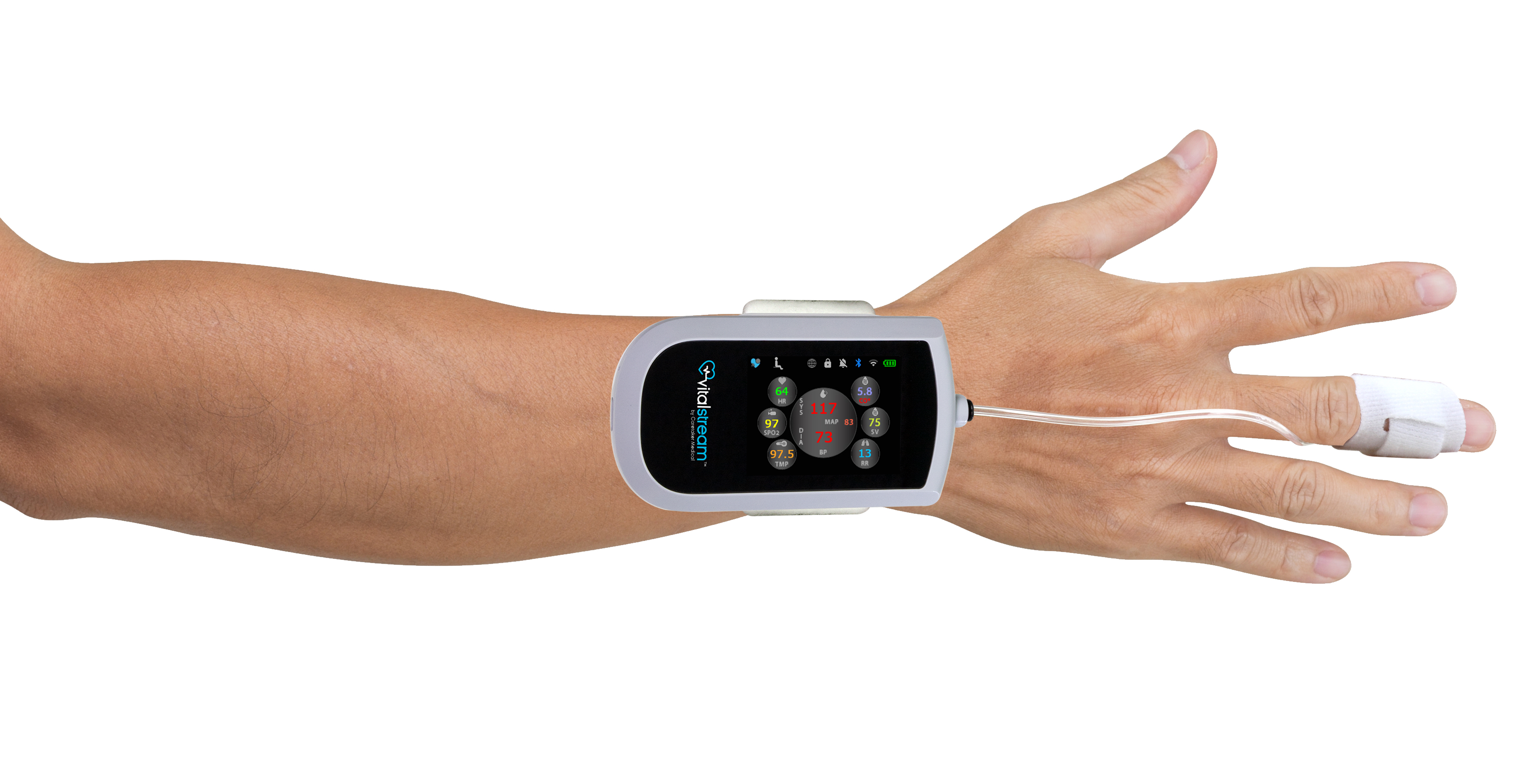
FDA Clears Caretaker Medical's Wireless Platform for Continuous