FDA May Reclassify ECT Devices
FDA May Reclassify ECT Devices
The FDA may reclassify electroconvulsive therapy devices from class III to class II. The FDA is accepting public comments on the change until March 28.
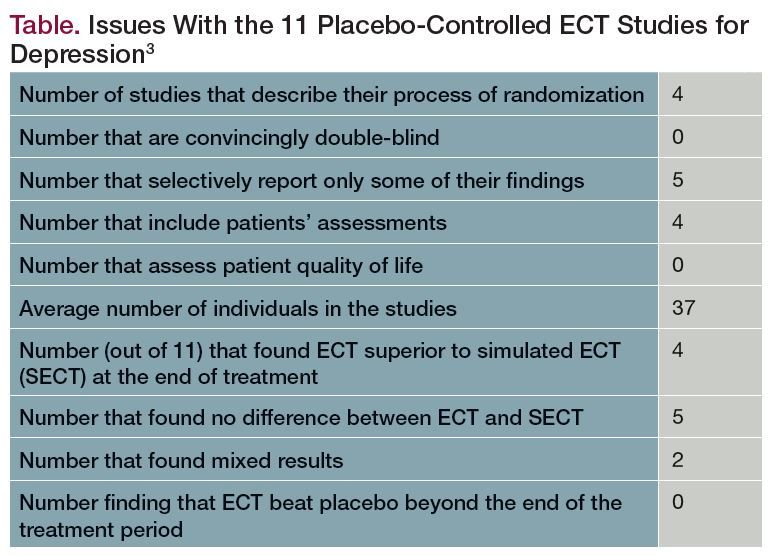
ECT: Dangerous on Either Side of the Pond
Left to Their Own Devices: Breakdowns in United States Medical

ECT Device Manufacturer Admits Brain Damage as a Risk of

Why the FDA's Move to Restrict ECT Should Alarm Us All
Psychiatrists Urged to Support ECT Reclassification

ECT Device Manufacturer Admits Brain Damage as a Risk of

FDA Downgrades Risk Category for Certain Uses - Psych News Alert

ECT Safety Reclassification: Psychiatrist Daniel P. Fisher to FDA

Electroconvulsive therapy - Wikipedia

F.D.A. Is Studying the Risk of Electroshock Devices

When to consider electroconvulsive therapy (ECT) - Kellner - 2020


