B'oh

B'oh
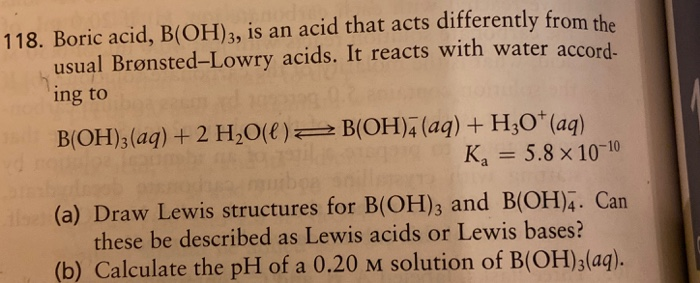
Solved 118. Boric acid, B(OH)3, is an acid that acts
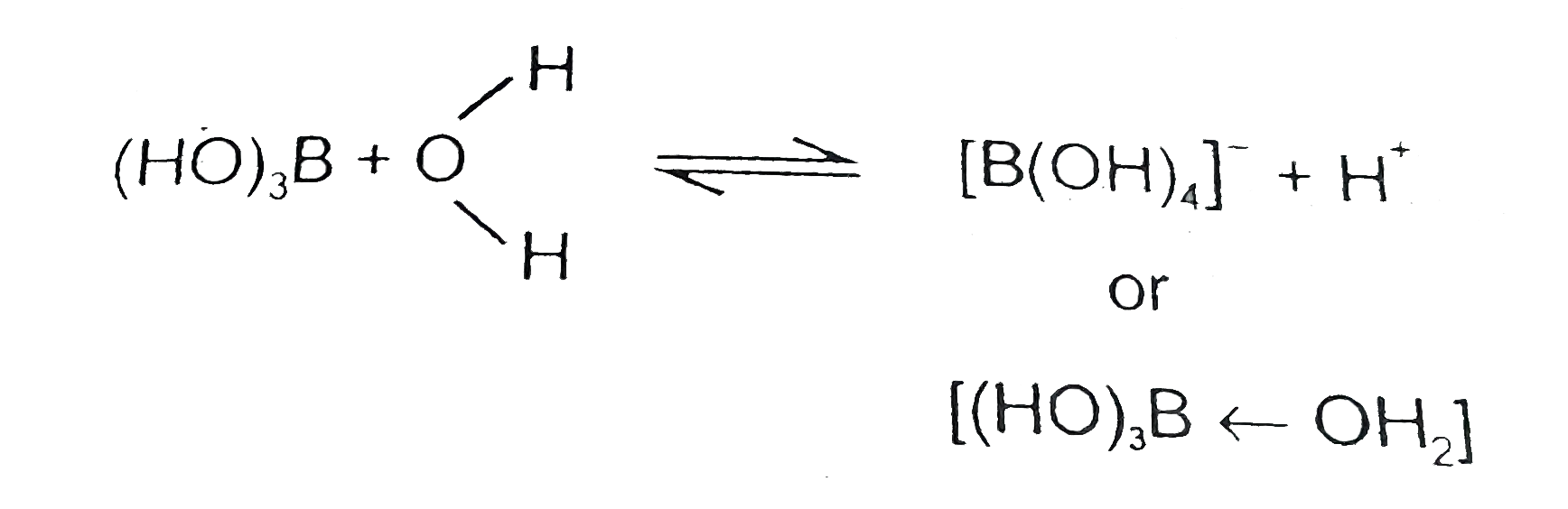
Although boric acid B(OH)(3), contains three hydroxyl groups yet it be

Hydroxyfluorooxoborate Na[B3O3F2(OH)2]⋅[B(OH)3]: Optimizing the

Thermodynamic and Transport Properties of H2/H2O/NaB(OH)4 Mixtures

Normalized FT-IR spectra of An-B(OH)2, HPG-β-CD and An-HPG-β-CD

7-Beta-Hydroxy-Dehydroepiandrosterone (7-B-OH-DHEA) Labeled d9
Boric acid, H3BO3

B'oh! : r/TheSimpsons
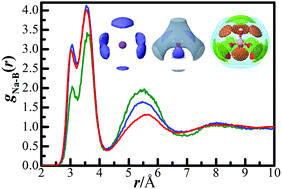
B(OH)4− hydration and association in sodium metaborate solutions
Why is B(OH) 3 or H3BO3 not a protonic acid? - Quora
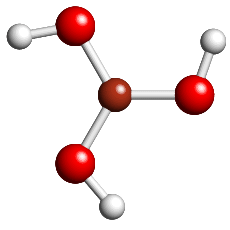
B(OH)3
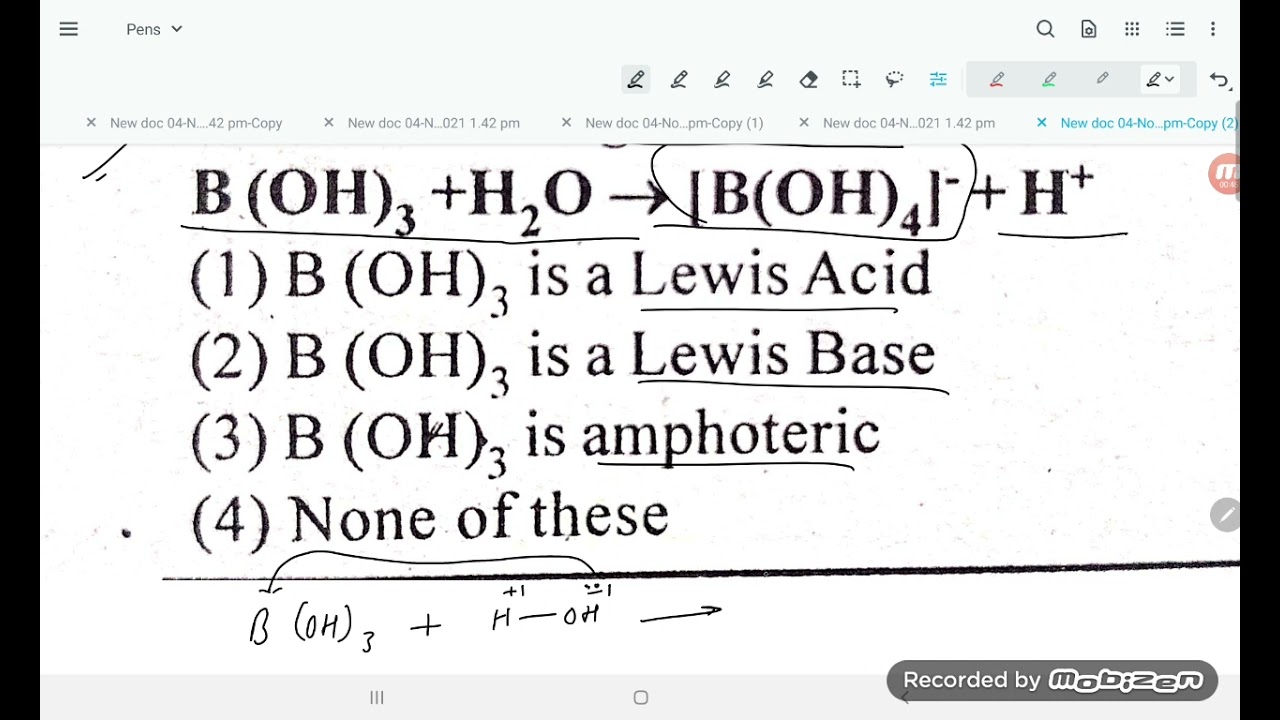
in the following reaction B(OH)3 + H2O gives [B(OH)4] + H+
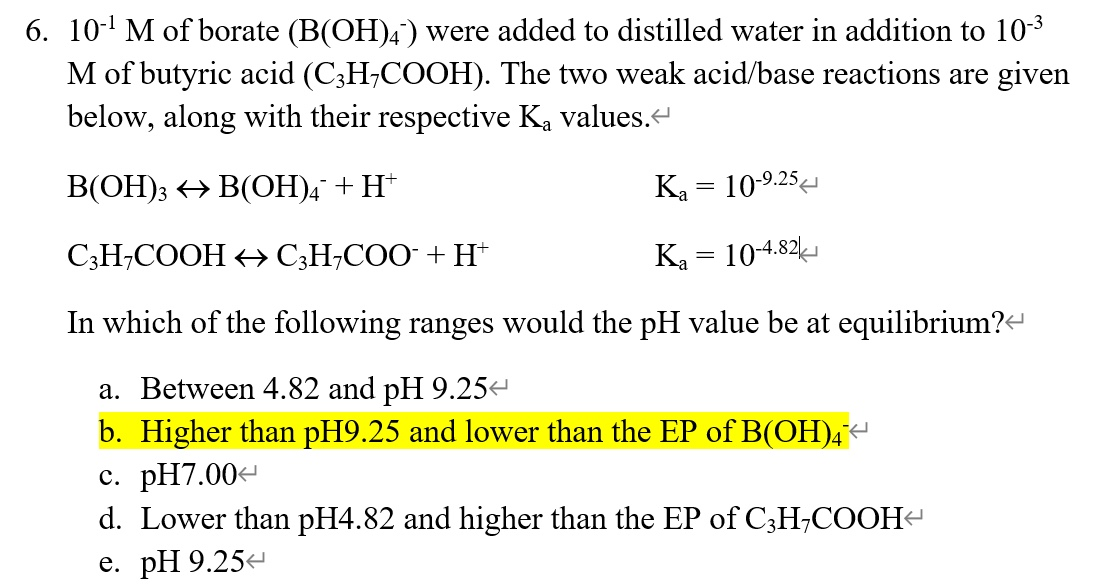
Solved 6. 10-1 M of borate (B(OH)4 ) were added to distilled

Base-Catalyzed Aryl-B(OH)2 Protodeboronation Revisited: From

![Sodium borate - Na2[B4O5(OH)4]�8H2O Structure, Molecular Mass Sodium borate - Na2[B4O5(OH)4]�8H2O Structure, Molecular Mass](https://cdn1.byjus.com/wp-content/uploads/2019/05/sodium-borate-structure.png)